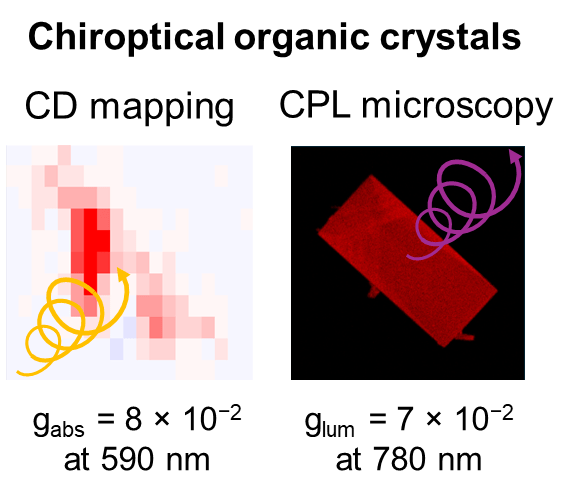
• Tuning Circular Dichroism and Circularly Polarised Luminescence in Single Crystals of a Perylene Diimide Macrocycle
D. Hartmann, S. E. Penty, A. Krimovs, R. Pal, T.-M. Gianga, G. Siligardi & T. A. Barendt
Angew. Chem. Int. Ed. 2026, e20567
The circular dichroism and circularly polarised luminescence of a bis-perylene diimide macrocycle are measured in single crystals. These chiroptical properties are tuned by π–π stacking interactions in the solid-state.
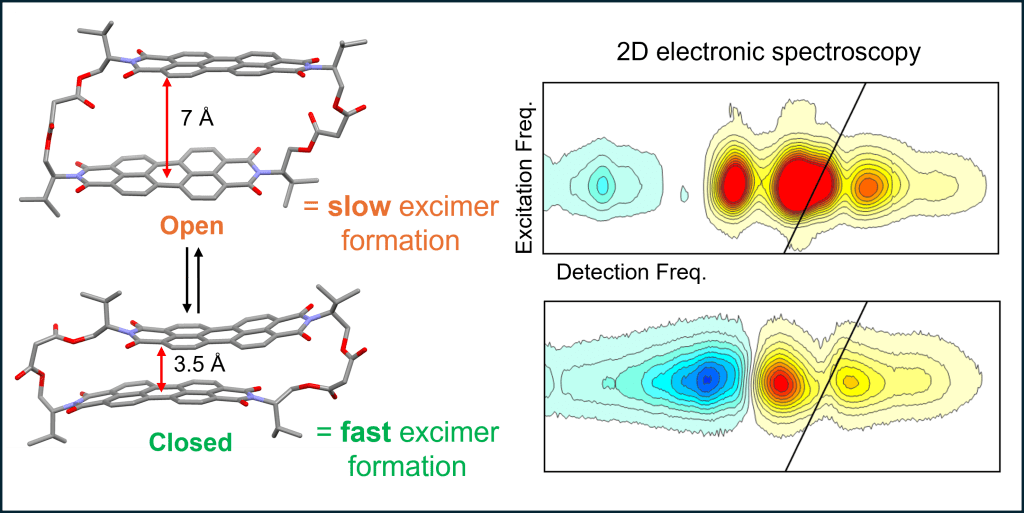
• Conformational Switching Modulates Excited-State Pathways in a Cofacial Perylene Dimer
G. Bressan, D. Hartmann, J. Brouwer, E. M Braun, J. N Bull & T. A. Barendt
Chem. Sci. 2026 doi.org/10.1039/D5SC09512C
Ultrafast spectroscopy uncovers conformation-dependent excimer population in a switchable bis-perylene diimide macrocycle.
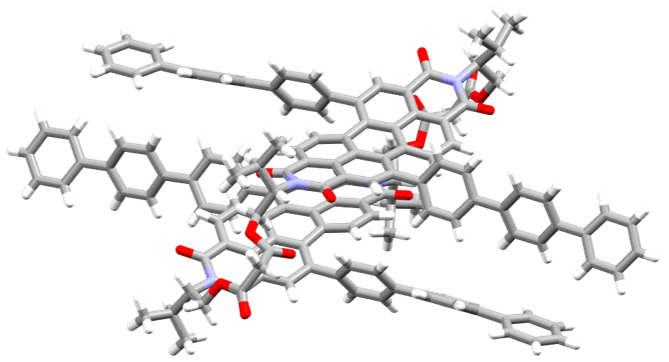
• Chirally locked and dynamic bis-perylene diimide macrocycles with multiple sources of chirality
D. Hartmann, S. E. Penty, R. Pal & T. A. Barendt
Commun. Chem. 2026 doi.org/10.1038/s42004-026-01904-z
We use a macrocycle to examine the impact of point, helical and supramolecular chirality on the chiroptical properties of a perylene diimide dimer.
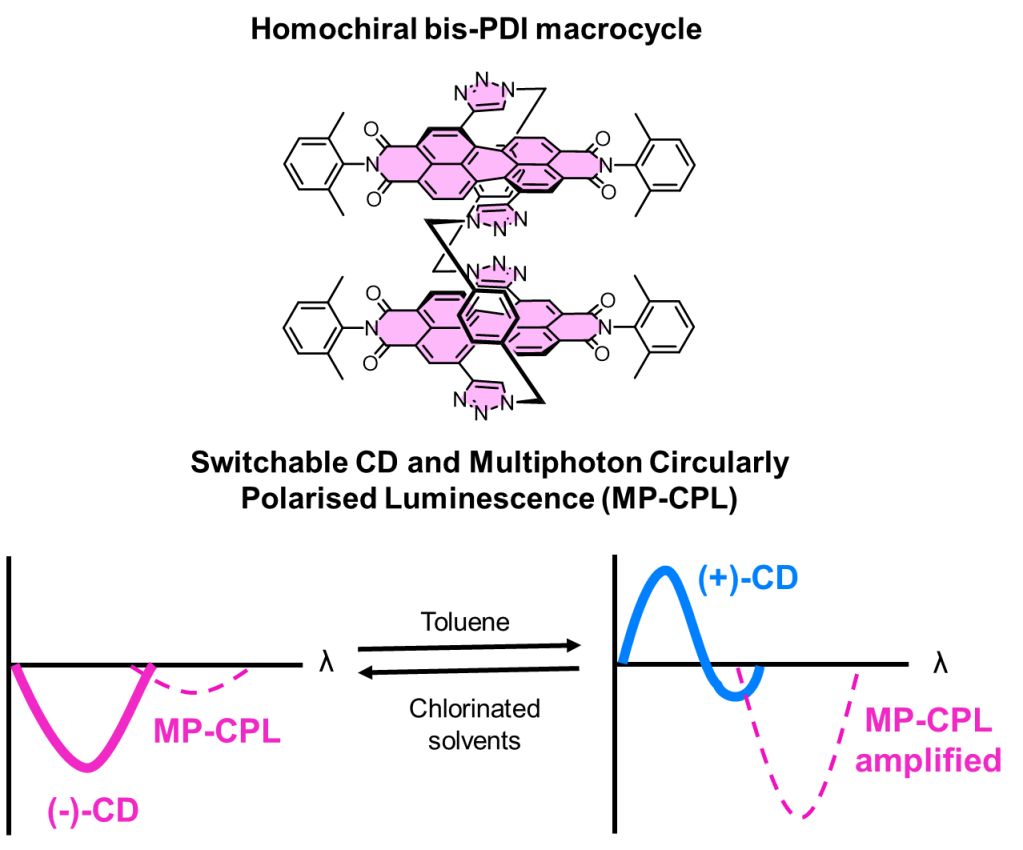
• Circular Dichroism and Multiphoton Circularly Polarized Luminescence Switching Using a Bis-perylene Diimide Macrocycle
S. E. Penty, M. V. Appleby, M. A. Zwijnenburg, D. J. Black, D. Hartmann, D. Chekulaev, J. Weinstein, R. Pal & T. A. Barendt
Chem. Eur. J., 2025, e01734
A chiral and configurationally stable bis-perylene diimide macrocycle exhibits switchable circular dichroism and multiphoton circularly polarized luminescence.
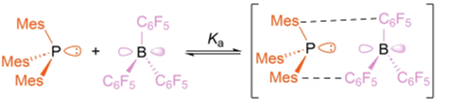
• Quantifying Interactions in the Active Encounter Complex of Frustrated Lewis Pairs
A. T. Littlewood, T. Liu, L. E. English, L. Chen, T. A. Barendt & A. R. Jupp
Nat. Comms., 2025, 16, 3666
Measuring the association constant of the active encounter complex of a frustrated Lewis pair.
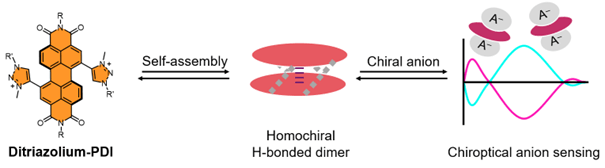
• Core-Twisted, Cationic Perylene Diimides; Homochiral Dimerisation and Chiroptical Anion Sensing
D. Hartmann, J. S. Hillis, L. E. Walker & T. A. Barendt
Chem. Eur. J., 2025, e202501270
A cationic organic dye forms a homochiral dimer for sensing chiral anions.
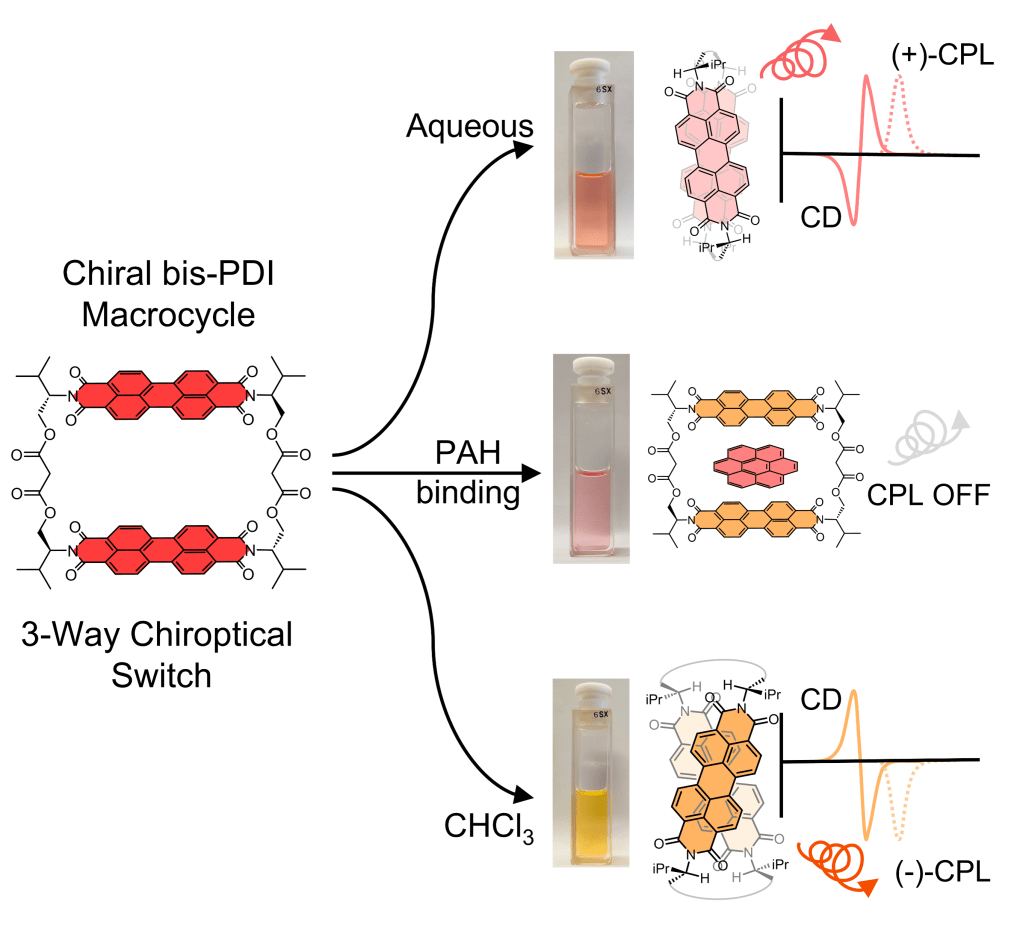
• A Bis-perylene Diimide Macrocycle Chiroptical Switch
D. Hartmann, S. E. Penty, M. A. Zwijnenburg, R. Pal & T. A. Barendt
Angew. Chem. Int. Ed. 2025, e202501122 – “Hot Paper“
A helical macrocycle that exhibits three-way switching of its chiroptical properties (+/−/off).
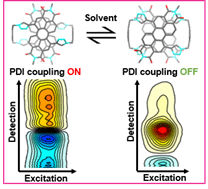
• Ultrafast and Coherent Dynamics in a Solvent Switchable “Pink Box” Perylene Diimide Dimer
G. Bressan, S. Penty, D. Green, I. Heisler, G. Jones, T. A. Barendt & S. R. Meech
Angew. Chem. Int. Ed. 2024, e202407242 – “Hot Paper“
A study of the perylene diimide dimer in the Pink Box using two-dimensional electronic spectroscopy.
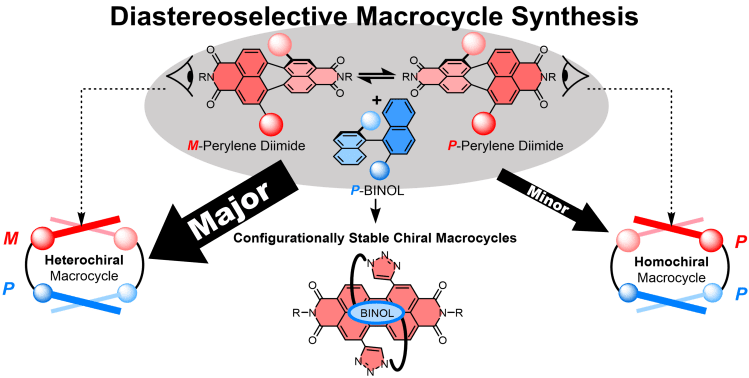
• Investigating the diastereoselective synthesis of a macrocycle under Curtin–Hammett control
A. Yeung, M. A. Zwijnenburg, G. R. F. Orton, J. H. Robertson & T. A. Barendt
Chem. Sci. 2024, 15, 5516-5524 – “Hot Article“
An invesitgation into the stereoselective synthesis of chiral macrocycles using the Curtin-Hammett principle.
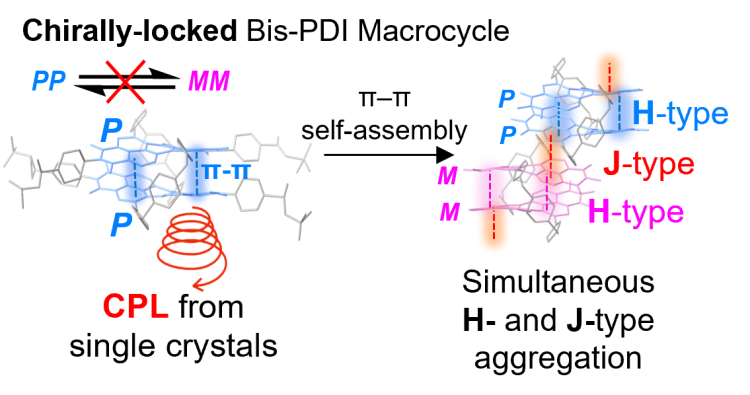
• A Chirally Locked Bis-perylene Diimide Macrocycle: Consequences for Chiral Self-Assembly and Circularly Polarized Luminescence
S. E. Penty, G. R. F. Orton, D. J. Black, R. Pal, M. A. Zwijnenburg & T. A. Barendt
J. Am. Chem. Soc. 2024, 146, 5470–5479
The chiral locking of a bis-perylene diimide macrocycle enables us to investigate chiral self-assembly and circularly polarised luminescence in single crystals.
• The Pink Box: Exclusive Homochiral Aromatic Stacking in a Bis-perylene Diimide Macrocycle
S. E. Penty, M. A. Zwijnenburg, G. R. F. Orton, P. Stachelek, R. Pal, Y. Xie, S. L. Griffin & T. A. Barendt
J. Am. Chem. Soc. 2022, 144, 12290-12298
A new chiral macrocycle exhibiting switchable conformations, red-shifted circularly polarised luminescence and other interesting chiroptical properties.

• Supramolecular Chemistry Primer
P. D. Beer, T. A. Barendt & J. Y. C. Lim
ISBN: 9780198832843
A primer text on the fudamentals and applications of Supramolecular Chemistry (published by OUP)

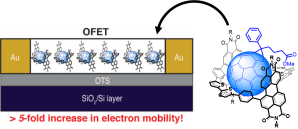
• Supramolecular Assemblies for Electronic Materials
T. A. Barendt, M. L. Ball, Q. Xu, B. Zhang, B. Fowler, A. Schattman, V. C. Ritter, M. L. Steigerwald & C. Nuckolls
Chem. Eur. J., 2020, 26, 3744-3748
The formation of a macrocycle-fullerene host-guest complex enhances electron mobility in the semiconducting active layer of an organic field effect transistor (OFET).
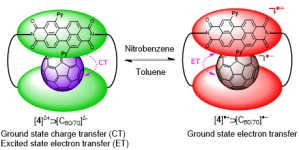
• The green box: an electronically versatile perylene diimide macrocyclic host for fullerenes
T. A. Barendt, W. K. Myers, S. P. Cornes, M. A. Lebedeva, K. Porfyrakis, I. Marques, V. Félix & P. D. Beer
J. Am. Chem. Soc., 2020, 142, 349-364
Supramolecular electron transfer to C60 fullerene when it is bound by a perylene diimide-based macrocycle nicknamed the “Green Box”.
• Anion mediated photophysical behavior in a C60 fullerene [3]rotaxane shuttle
T. A. Barendt, I. Rašović, M. A. Lebedeva, G. A. Farrow, A. Auty, D. Chekulaev, I. V. Sazanovich, J. A. Weinstein, K. Porfyrakis & P. D. Beer
J. Am. Chem. Soc., 2018, 140, 1924-1936
Photoinduced electron transfer in an interlocked donor–acceptor system is controlled by anion induced molecular motion.
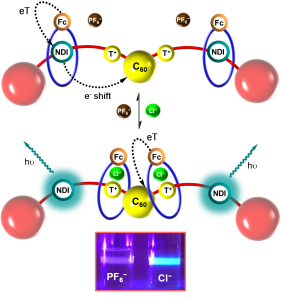
• Anion- and solvent-induced rotary dynamics and sensing in a perylene diimide [3]catenane
T. A. Barendt, L. Ferreira, I. Marques, V. Félix & P. D. Beer
J. Am. Chem. Soc., 2017, 139, 9026-9037
Unprecedented anion-induced circumrotatory motion in a perylene-diimide hetero[3]catenane, enables optical anion sensing.
• Selective nitrate recognition by a halogen bonding [3]rotaxane molecular shuttle.
T. A. Barendt, A. Docker, I. Marques, V. Félix & P. D. Beer
Angew. Chem. Int. Ed., 2016, 55, 11069 –11076
A halogen bonding [3]rotaxane, capable of colourimetric oxoanion sensing courtesy of pincer-like molecular motion.
• Superior anion-induced shuttling behaviour exhibited by a halogen bonding two station rotaxane.
T. A. Barendt, S. Robinson & P. D. Beer
Chem. Sci., 2016. 7, 5171-5180
Halogen bonding anion recognition and aromatic stacking interactions control molecular shuttling in a bistable [2]rotaxane.
• Halogen bonding in supramolecular chemistry
L. C. Gilday, S. W. Robinson, T. A. Barendt, M. J. Langton, B. R. Mullaney & P. D. Beer
Chem. Rev., 2015, 115, 7118–7195
Comprehensive review of halogen bonding across all aspects of supramolecular chemistry.
• Anion sensing by solution- and surface-assembled osmium(II) bipyridyl rotaxanes
J. Lehr, T. Lang, O. A. Blackburn, T. A. Barendt, S. Faulkner, J. J. Davis & P. D. Beer
Chem. Eur. J. 2013, 19, 15898-15906
Optical and electrochemical anion sensing in solution and on a surface using a transition metal-appended [2]rotaxane host.