Supramolecular Chemistry of Organic Dyes
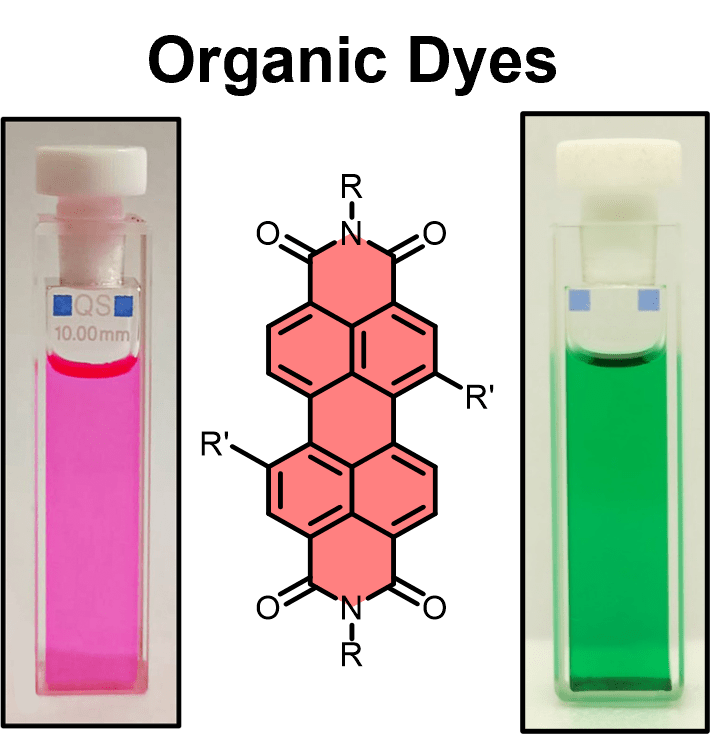
We are a synthetic organic chemistry research group working in supramolecular chemistry, a field concerning the non-covalent interactions between molecules. We primarily study interactions involving colourful organic dyes such as perylene diimides, molecules which have applications across organic (opto)electronics, chemical sensing and materials chemistry.
Specific themes within our research include synthetic macrocycles, molecular recognition and self-assembly and mechanically interlocked molecules.
Synthetic macrocycles
Molecular recognition & self-assembly
Mechanically interlocked molecules
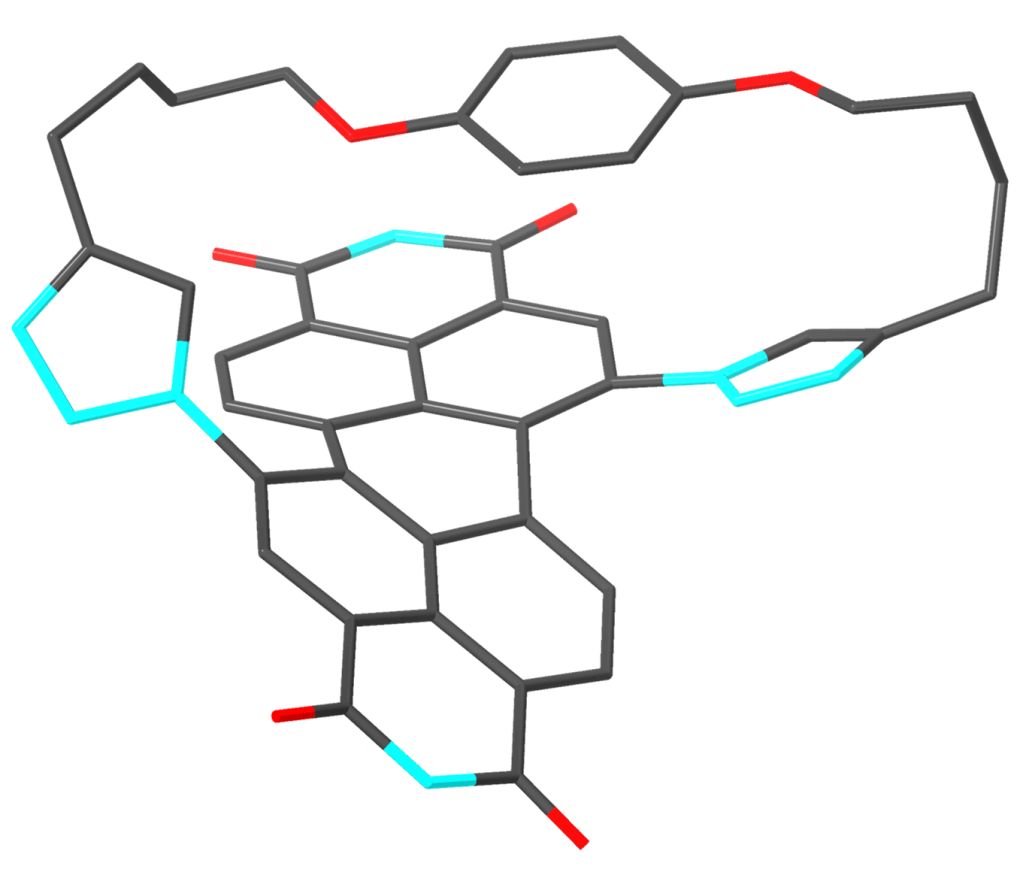
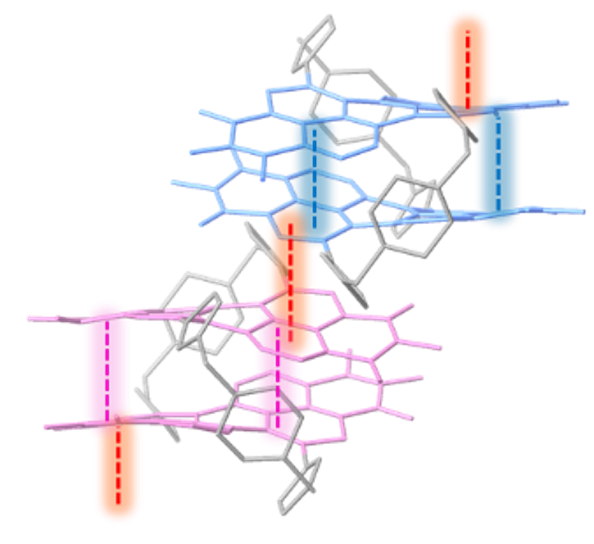
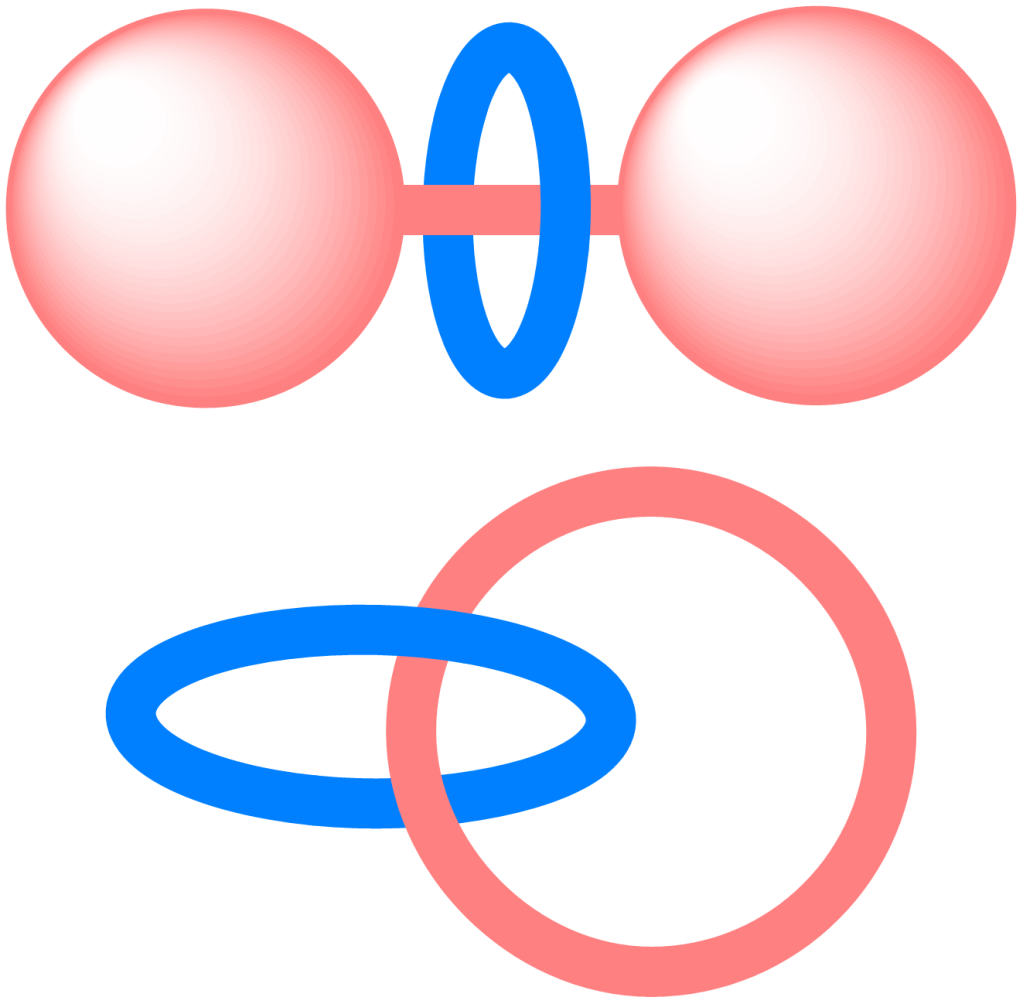
Macrocycles provide preorganised scaffolds to probe non-covalent interactions between aromatic functional groups.
E.g. Chem. Sci. 2024, 10.1039/D3SC05715A
The interactions beteween organic dyes can be used for guest binding or to direct their supramolecular self-assembly.
E.g. J. Am. Chem. Soc. 2024, 146, 5470–5479
Rotaxanes and catenanes can be used to control the dynamics of dye-based molecular components.
E.g. J. Am. Chem. Soc., 2018, 140, 1924-1936
To support this research we are fortunate to collaborate with a number of excellent scientists, including Prof Martijn Zwijnenburg (UCL), Prof Robert Pal (Durham), Dr Georgia Orton (Birmingham) and Dr Andy Jupp (Birmingham).
Synthetic macrocycles
We design, synthesise and analyse new macrocycles containing twisted (i.e. chiral) dyes such as perylene diimides.
For example, our bis-perylene diimide macrocycle, known as the “Pink Box” due to its colour, can be used to tune the electronic communication between two chiral dyes and so investigate the impact this coupling has on chiral, photophysical and electrochemical properties: J. Am. Chem. Soc. 2022, 144, 12290-12298
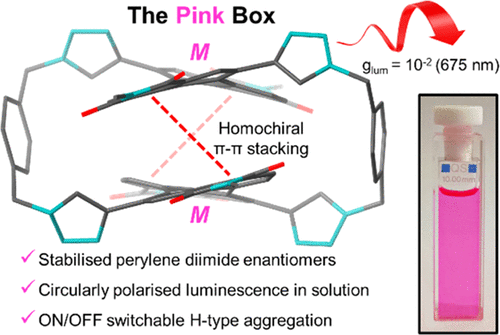
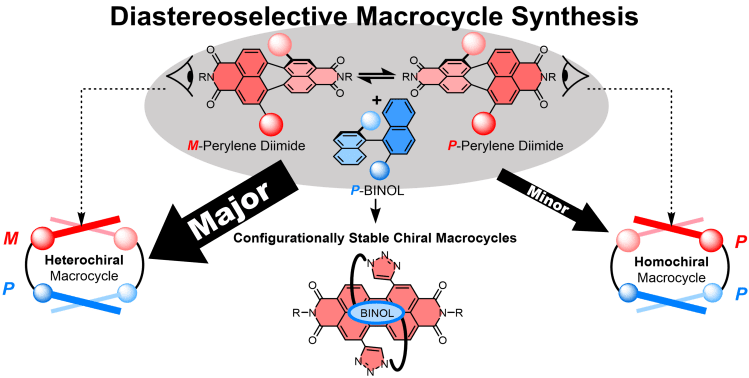
We are also investigating the stereoselective synthesis of such macrocycles by using templating non-covalent interactions between the aromatic components: Chem. Sci. 2024, 10.1039/D3SC05715A
Molecular recognition and self-assembly
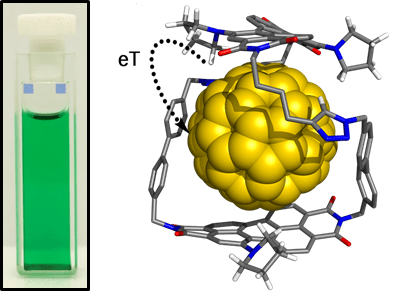
Organic dye-based macrocycles may be used for binding aromatic guest molecules. For example, the macrocycle shown below, the Green Box, encapsulates a C60 fullerene guest inside its π-electron rich cavity, to promote intermolecular electron transfer: J. Am. Chem. Soc., 2020, 142, 349-364
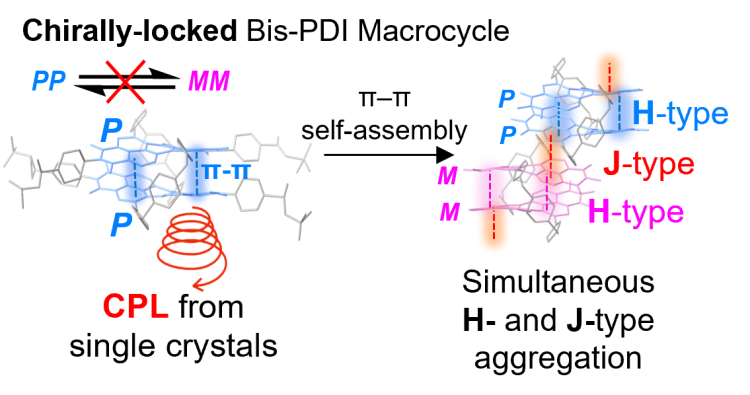
By locking the chirality of perylene diimide dyes we are able to investigate the impact of chirality on their π-π self-assembly in both solution and single crystals which is important for new chiroptical materials: J. Am. Chem. Soc. 2024, 146, 5470–5479
Mechanically interlocked molecules
Mechanically interlocked molecules (MIMs) such as catenanes and rotaxanes enable control over molecular motions since their degrees of freedom are restricted by the mechanical bond.
Here, a [3]rotaxane molecular shuttle has been used to control photoinduced electron transfer between an electron donor (Fc = ferrocene) and electron acceptor groups (naphthalene diimide = NDI or fullerene = C60) groups: J. Am. Chem. Soc., 2018, 140, 1924-1936
